IPR’s automation products help achieve FDA compliance by offering features such as UL508A certified control panels, configurable security access, Active Directory Domain integration, electronic signatures. audit trail, reporting, OPC interoperability, and MES interface.
Tablet Press Weight Control & Reject Systems
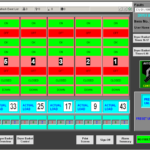
Find out more about the available upgrade options on your tablet press for automatic tablet weight control and reject capabilties. Options include single tablet reject with verification, in-tablet testing and feedback, audit trail, electronic signature, product recipes, and batch reporting.
Tablet Press Compression Force Monitoring & Data Logging
Contact IPR to learn about the available compression force monitoring and data logging options available on your tablet press from a simple graphical force meter to wave form compression display. Capture the data for offline analyis and trending.
New Machine Wiring & Controls
Overall Equipment Efficiency